Ideal Gas Law Calculator
Use our Ideal Gas Law Calculator to calculate pressure, volume, temperature, and the number of moles in an ideal gas. This easy-to-use tool simplifies your gas law calculations.
Ever wondered how scientists figure out the volume of a gas or its pressure in a container? That’s where the Ideal Gas Law comes in! It’s a simple yet powerful equation that helps us understand how gases behave under different conditions. And guess what? You don’t need to be a scientist to use it. With our Ideal Gas Law Calculator, solving gas problems is a breeze!
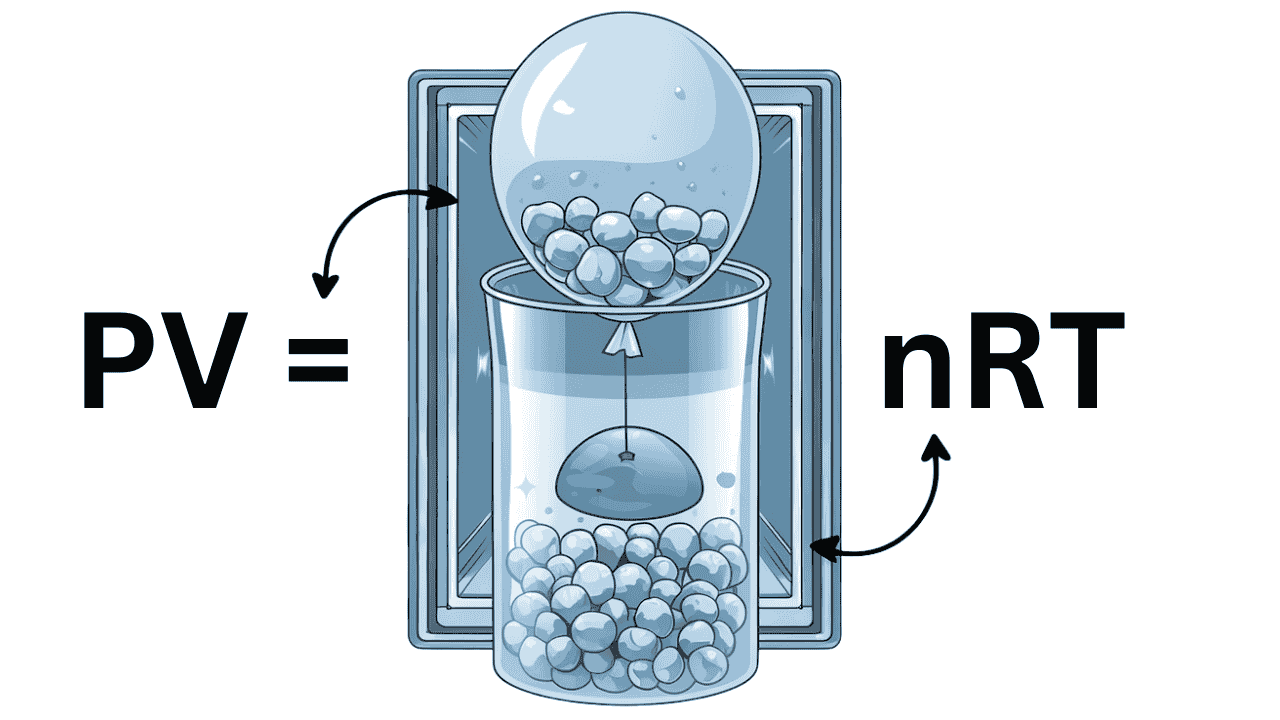
What Is the Ideal Gas Law?
The Ideal Gas Law is an equation that links four key properties of a gas:
PV = nRT
Let’s break that down:
- P = Pressure (in atm or Pascals)
- V = Volume (in liters or cubic meters)
- n = Number of moles of gas
- R = Gas constant (0.0821 L·atm/mol·K or 8.314 J/mol·K)
- T = Temperature (in Kelvin)
This equation is super flexible! If you know three of these values, you can calculate the fourth. That’s what makes it so useful.

Why Does It Matter?
The Ideal Gas Law helps with:
✔️ Finding the pressure of a gas at a given temperature and volume.
✔️ Calculating how much space a gas will take up.
✔️ Determining the number of gas molecules in a sample.
It’s widely used in chemistry, physics, and even engineering. Whether you're in a lab or just curious about how gases work, this equation has you covered!
How It Works in Real Life
Gases don’t always behave perfectly, but this law gives a good estimate in most cases. When does it work best? When the gas is at:
✅ Normal temperatures
✅ Low to moderate pressures
At extreme conditions (like super high pressures or ultra-cold temperatures), real gases may not follow the Ideal Gas Law exactly. But for everyday calculations? It’s spot on!
How to Use the Ideal Gas Law Calculator
Using our calculator is easy. Just follow these steps:
- Enter what you know – Plug in the values you have for P, V, T, or n.
- Leave one blank – The missing value is what we’ll calculate for you.
- Hit ‘Calculate’ – The answer pops up instantly!
It’s like having a mini scientist in your pocket!
Example Calculation
Let’s say you need to find the volume of a gas with these conditions:
- Pressure = 2 atm
- Temperature = 300 K
- Moles of gas = 1
- R = 0.0821 L·atm/mol·K
We rearrange the formula to solve for V:
V = (nRT) / PV = (1 × 0.0821 × 300) / 2V = 12.315 liters
So, the gas takes up 12.315 L of space!
Formula Chart Sheet
Want a quick way to rearrange the equation? Use these formulas:
- Pressure (P): P = (nRT) / V
- Volume (V): V = (nRT) / P
- Moles (n): n = PV / RT
- Temperature (T): T = PV / nR
Just plug in your numbers and solve!
Final Thoughts
The Ideal Gas Law Calculator makes solving gas problems a snap. Whether you’re a student, a professional, or just someone who loves science, it’s a must-have tool. With just a few clicks, you can solve tricky equations and understand how gases behave.
FAQs
What’s the Ideal Gas Law in simple terms?
It’s a formula (PV = nRT) that shows how gases behave based on pressure, volume, temperature, and the number of molecules.
How do I calculate pressure?
Use P = (nRT) / V where n = moles, R = gas constant, T = temperature, and V = volume.
What units should I use?
- Pressure: atm or Pa
- Volume: liters or cubic meters
- Temperature: Kelvin (K)
- Moles: mol
- Gas constant (R): 0.0821 L·atm/mol·K or 8.314 J/mol·K
When does the Ideal Gas Law not work well?
At very high pressures or super low temperatures, real gases don’t behave ideally. But for most everyday situations, it’s pretty accurate!
How do I find volume?
Use V = (nRT) / P and plug in your values.
What’s the gas constant (R)?
It’s a number that makes the equation work, with values like 0.0821 L·atm/mol·K or 8.314 J/mol·K.